Spermidine
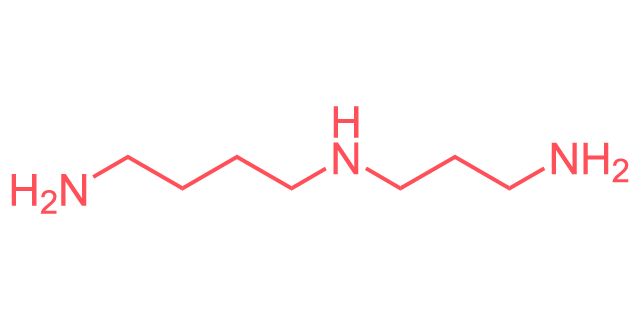
Linear Formula
C7H19N3
Synonyms
n/a
Share this metabolite
Spermidine is an aliphatic polyamine that is synthesized from its precursor putrescine through the enzyme spermidine synthase1. Spermidine is present in all living cells and plays a critical role in maintaining cellular homeostasis. Its primary function is to engage cytoprotective autophagy, and it affects several processes, including cell growth, tissue regeneration, and DNA/RNA stabilization. Spermidine also possesses anti-inflammatory and antioxidant properties.
Spermidine is endogenously produced in mammalian cells throughout the body and can also be synthesized by the gut microbiome2. Exogenously, spermidine is found in food items, particularly unprocessed plant-derived foods (e.g., cereals, legumes, vegetables, fruits) and fermented items (e.g., mature cheese or soybean products)3. Dietary spermidine is rapidly reabsorbed from the intestines and distributed throughout the body. Systemic spermidine levels can be modulated directly through diet or indirectly through dietary effects on spermidine-producing bacteria4.
Due to spermidine’s cytoprotective effects, its impact has been observed across several age-related diseases and physiological processes. Interestingly, spermidine supplementation is strongly implicated in promoting longevity by mitigating the harmful consequences of cancer, metabolic syndrome, and cardiovascular diseases5.
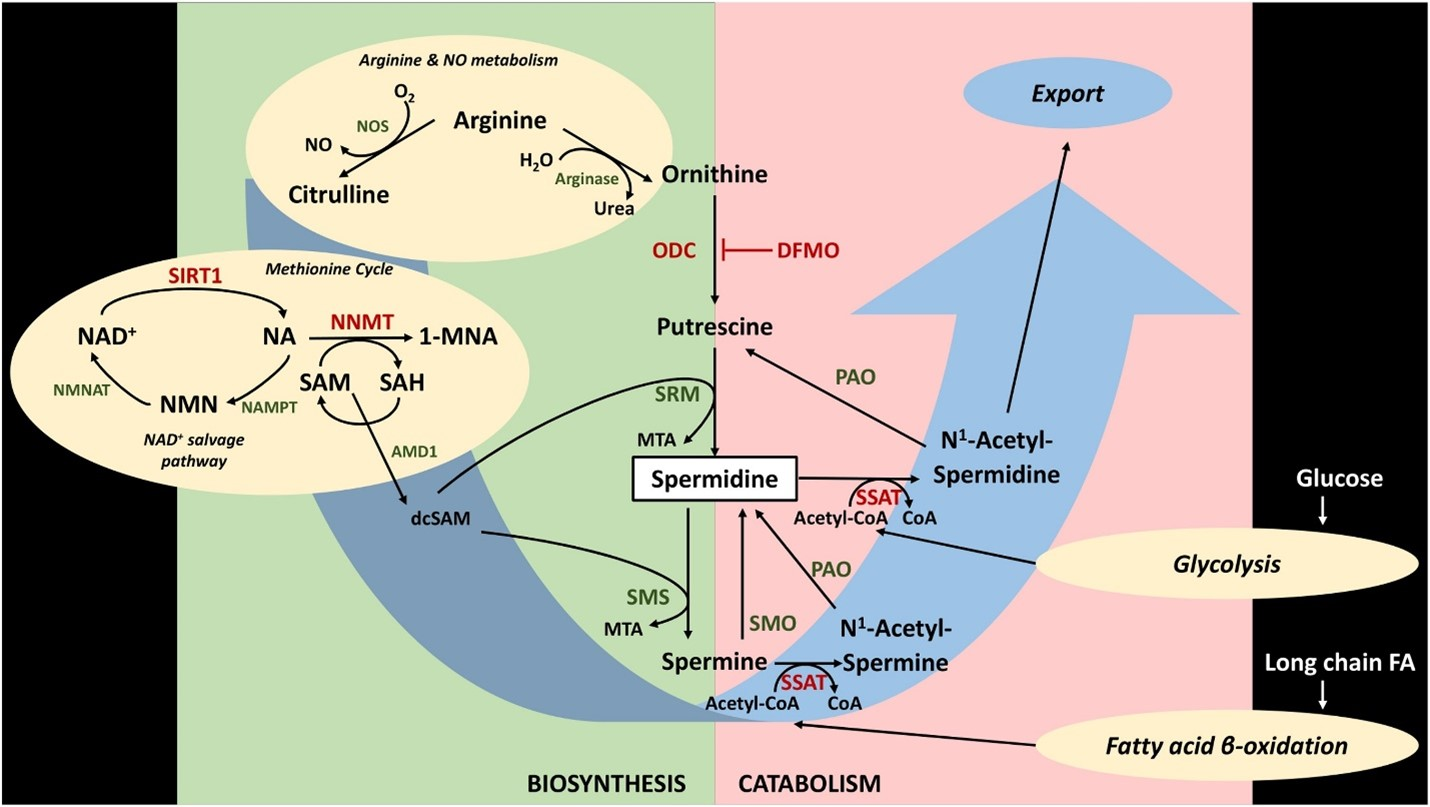
(Figure from Choksomngam et al., 20216)
Spermidine and Metabolic Health
In general, spermidine has protective effects on cardiovascular and metabolic health. However, there are some inconsistencies in epidemiological studies. For example, one report indicated that consumption of polyamine-rich food was associated with a lower incidence of cardiovascular disease7, while others have reported increased polyamine levels in metabolic syndrome patients with Type 2 diabetes8. Scientists hypothesize that this discrepancy is associated with the disease stage, where spermidine levels are elevated in more severe cases6.
Regardless, mechanistic examinations of spermidine are in support of its cytoprotective effects. Studies in aging mice demonstrated that spermidine supplementation improves diastolic function, ventricular elasticity, and mitochondrial function9. In another report that examined mice fed a high-fat diet, administration of spermidine resulted in weight loss, improved glucose utilization, and prevented adiposity10. In support of these data, others have demonstrated that the autophagy induced by spermidine is critical for its protective effects against weight gain, lipotoxicity, and pancreatic β-cell death11.
Spermidine and Gastrointestinal Health
A major source of endogenous spermidine is intestinal bacteria. With a wealth of literature implicating the gut microbiome and gastrointestinal health, polyamines like spermidine have been shown to have protective effects for gastrointestinal health, including maintenance of gut barrier function and reducing inflammation.
In a study involving diet-induced obese mice, spermidine supplementation improved metabolic parameters, an effect associated with the enhancement of gut barrier function and the increase of beneficial short-chain fatty acid producing bacteria12. Another report examining a mouse model of inflammatory bowel disease (IBD) demonstrated that spermidine supplementation promoted anti-inflammatory macrophages and prevented the shift toward a dysbiotic microbiome13.
Spermidine and Neuroscience
Polyamines like spermidine are also critical for maintaining central nervous system (CNS) function. Spermidine in the CNS helps mediate nerve growth and regeneration, responds to neuronal injury, and helps regulate neuronal ion channels14. Early studies demonstrated that individuals with Alzheimer’s disease exhibit abnormally elevated levels of spermidine, while more recent studies have shown that cognitive decline associated with Alzheimer’s disease is linked to a decrease in spermidine levels15. Interestingly, studies have shown that age-related cognitive decline in Drosophila can be ameliorated with spermidine supplementation, which prevents adverse changes in synaptic signaling16.
Spermidine’s neuroprotective effects can also be observed across other neurodegenerative disorders. For example, spermidine supplementation in mouse models of multiple sclerosis slows disease progression and mitigates the loss of retinal ganglion cells17.
Spermidine and Drug Development
Due to its cytoprotective and anti-aging effects, spermidine has generated considerable attention in therapeutics and drug development. Indeed, spermidine is currently being investigated as a treatment for cognitive decline and Alzheimer’s disease in a phase IIIb clinical trial18. Moreover, since systemic spermidine levels are altered across many diseases, it is also useful as a biomarker and target for therapeutics.
In developing countries with a high prevalence of tropical diseases caused by parasitic protozoa (e.g., Sleeping sickness), spermidine and other polyamine analogs are effective treatments for many parasite-borne diseases, with new polyamine-based treatments in development19. With an eye towards maximizing life span, polyamine therapeutics and spermidine intake are emerging as promising candidates for drugs designed to treat cancer, metabolic disease, cardiovascular disease, and neurodegeneration.
Spermidine in research
As of July 2023, there are over 14,000 citations for “spermidine” in research publications (excluding books and documents) on Pubmed. The vast number of publications linking this metabolite to metabolic and gastrointestinal health (including the microbiome component) suggests that any research program seeking to better understand gut and metabolic disorders may benefit from quantitative analysis of spermidine. Similarly, researchers may consider adding spermidine quantification to academic and preclinical research efforts focused on gut, metabolic, and neurological health.
References
- Raina A and Janne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol 1975;(53):121-147.
- Matsumoto M, Kibe R, Ooga T et al. Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep 2012;(2):233.
- Munoz-Esparza NC, Latorre-Moratalla ML, Comas-Baste O et al. Polyamines in Food. Front Nutr 2019;(6):108.
- Milovic V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol 2001;(13):1021-1025.
- Madeo F, Bauer MA, Carmona-Gutierrez D et al. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019;(15):165-168.
- Choksomngam Y, Pattanakuhar S, Chattipakorn N et al. The metabolic role of spermidine in obesity: Evidence from cells to community. Obes Res Clin Pract 2021;(15):315-326.
- Soda K, Kano Y, and Chiba F. Food polyamine and cardiovascular disease–an epidemiological study. Glob J Health Sci 2012;(4):170-178.
- Fernandez-Garcia JC, Delpino-Rius A, Samarra I et al. Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case(-)Control Study from the PREDIMED-Plus Trial. J Clin Med 2019;(8).
- Eisenberg T, Abdellatif M, Schroeder S et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 2016;(22):1428-1438.
- Sadasivan SK, Vasamsetti B, Singh J et al. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur J Pharmacol 2014;(729):94-99.
- Fernandez AF, Barcena C, Martinez-Garcia GG, et al. Autophagy counteracts weight gain, lipotoxicity and pancreatic beta-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis 2017;(8):e2970.
- Ma L, Ni Y, Wang Z, et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020;(12):1-19.
- Niechcial A, Schwarzfischer M, Wawrzyniak M et al. Spermidine ameliorates colitis via induction of anti-inflammatory macrophages and prevention of intestinal dysbiosis. J Crohns Colitis 2023
- Gilad GM and Gilad VH. Early polyamine treatment enhances survival of sympathetic neurons after postnatal axonal injury or immunosympathectomy. Brain Res 1988;(466):175-181.
- Xu J, Sun Z, Zhang R, et al. Non-linear association between serum spermidine and mild cognitive impairment: Results from a cross-sectional and longitudinal study. Front Aging Neurosci 2022;(14):924984.
- Gupta VK, Pech U, Bhukel A et al. Spermidine Suppresses Age-Associated Memory Impairment by Preventing Adverse Increase of Presynaptic Active Zone Size and Release. PLoS Biol 2016;(14):e1002563.
- Guo X, Harada C, Namekata K et al. Spermidine alleviates severity of murine experimental autoimmune encephalomyelitis. Invest Ophthalmol Vis Sci 2011;(52):2696-2703.
- Wirth M, Schwarz C, Benson G et al. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)-study protocol for a randomized controlled trial. Alzheimers Res Ther 2019;(11):36.
- Reguera RM, Tekwani BL, and Balana-Fouce R. Polyamine transport in parasites: a potential target for new antiparasitic drug development. Comp Biochem Physiol C Toxicol Pharmacol 2005;(140):151-164.

