Case Study
Metabolomics uncovers time-dependent metabolic responses to exercise
In collaboration with Metabolon, leading researchers reveal dynamic metabolic responses across key tissues that are modulated depending on timing of exercise.
A recent study published in Cell Metabolism used Metabolon’s Global Discovery Panel to characterize the metabolic responses to exercise timing in nine different tissue types collected from mice. They identified several key metabolites associated with both active and rest periods. These results are a starting point for a unraveling the relationship between exercise and energy homeostasis and how disrupted relationships are linked to metabolic disease.
A recent study published in Cell Metabolism used Metabolon’s Global Discovery Panel to characterize the metabolic responses to exercise timing in nine different tissue types collected from mice. They identified several key metabolites associated with both active and rest periods. These results are a starting point for a unraveling the relationship between exercise and energy homeostasis and how disrupted relationships are linked to metabolic disease.

The Challenge
Circadian rhythms are critical for maintaining metabolic homeostasis, and disruption of circadian alignment can lead to metabolic diseases. Timing of food intake can change circadian coordination of metabolic responses and subsequent disease progression. Similarly, temporal control of exercise can also impact energy homeostasis and metabolic pathways relevant for glycemic control and weight loss. However, the optimal timing of exercise required to generate desired metabolic outcomes across various tissues remains unclear. To tackle this challenge, Metabolon helped scientists uncover and map global metabolite responses to exercise across tissues associated with metabolic health1.
The Metabolon Insight
To understand how exercise timing impacts tissue and systemic metabolism, scientists compared samples obtained from mice that performed for one hour on a treadmill when they typically sleep (rest phase) or are typically awake (active phase), as well as sedentary controls. Comprehensive sample collection included serum, skeletal muscle, liver, heart, hypothalamus (HT), epididymal white adipose tissue (eWAT), inguinal white adipose tissue (iWAT), and interscapular brown adipose tissue (BAT) sample for metabolomics analysis.
Using untargeted metabolomics, scientists identified 600-900 metabolites in each tissue type including ~550-800 annotated metabolites. Further analyses of these data not only revealed differences in exercise time and group (exercise vs. sedentary), but also unique metabolic responses depending on the time of day exercise was performed. For example, exercise during the active phase altered more muscle, serum, heart, HT, iWAT, and BAT metabolites compared to the rest phase. Moreover, altered metabolites exhibit time-dependent dissociations – eWAT metabolites generally decreased with exercise in the rest phase, but increased during the active phase.
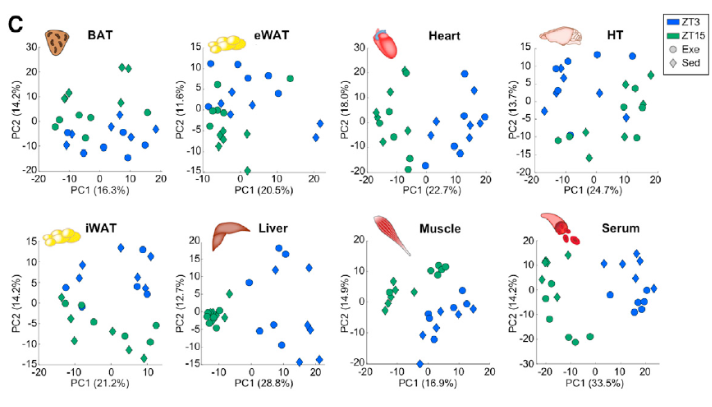
Figure 1: Metabolomics reveals time and tissue-dependent responses to exercise.
With a global metabolomic profile, scientists were able to dissect these data into metabolite classes. Interestingly, amino acids (AA) and lipids were more impacted by exercise during the active phase, but these changes were also dependent on tissue type. For example, fatty acid and glycerol metabolites accumulated in the liver following exercise during the rest phase while the same metabolites accumulated in serum and iWAT following active phase exercise. A number of other metabolites (e.g. corticosterone, glycogens, nucleotides, carbohydrates) also exhibited tissue and time-dependent effects of exercise.
Applying Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis to their dataset, the scientist found robust time-of-day effects of exercise on pathway molecules associated with nucleotides (e.g. purine, pyrimidine), AAs (e.g. glycine, serine, and threonine) or fatty acids (e.g. unsaturated fatty acid biosynthesis metabolites). Moreover, pair-wise metabolite correlations revealed strong time-dependent crosstalk between muscle and liver, with particularly strong effects during active phase exercise. Their map of exercise metabolism also provided improved mechanistic insight into exerkines (signaling metabolites that respond to exercise) such as 2-hydroxybutyrate (2-HB), which exhibits dynamic fluctuations depending on time of day and is robustly modulated by exercise during the active phase.
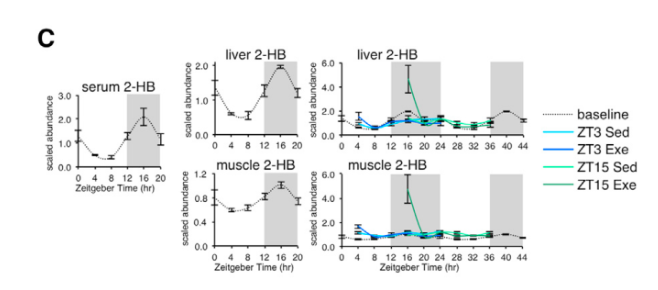
Figure 2: Exerkines such as 2-HB exhibit temporal responses to circadian rhythms and are modulated by exercise time.
The Value of Discovery
By leveraging the insights provided by global untargeted metabolomics data, scientists have gained greater clarity and context on energy homeostasis. Given the sensitivity of metabolic responses to circadian rhythms, the research presented here reveals an “atlas of exercise metabolism” that is robustly impacted by the time of day. Together, these scientists compiled an important resource that serves as a starting point for a comprehensive understanding of a variety of signaling metabolites linked to metabolic diseases and uncovers mechanistic insights toward optimizing the benefits of exercise.
References
1. Sato, S., Dyar, K.A., Treebak, J.T., Jepsen, S.L., Ehrlich, A.M., Ashcroft, S.P., Trost, K., Kunzke, T., Prade, V.M., Small, L., et al. (2022). Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab 34, 329-345 e328. 10.1016/j.cmet.2021.12.016.