Case Study
Dogs With Mitral Valve Disease Have Bioenergetic and Metabolic Readaptations and Reduced Kidney Function
Metabolomics identifies changes in key metabolic pathways in the development of canine myxomatous mitral valve disease and congestive heart failure.
The Metabolon Global Discovery Panel identified changes in metabolic pathways and biomarkers with potential clinical utility in canine heart disease.
Metabolon Discovery: Global Platform identified changes in metabolic pathways and biomarkers with potential clinical utility in canine heart disease.

The Challenge: Understanding Mitral Valve Disease Mechanism
Myxomatous mitral valve disease (MMVD) is the most common naturally acquired heart disease in dogs,1-3 occurring with greater frequency in geriatric small- and medium-breeds.3,4
In MMVD, the mitral valve thickens, preventing complete closure of the valve, allowing blood to flow backward into the left atrium (mitral regurgitation). The leak progressively worsens over time, causing increased pressure within the heart resulting in atrium and ventricles enlargement (referred to as cardiac remodeling).3,5 Cardiac remodeling leads to inefficient pumping of blood, eventually resulting in congestive heart failure (CHF) when the heart is no longer able to compensate.3,6-8 Dogs with MMVD are clinically classified according to the American College of Veterinary Internal Medicine staging system (stages A, B1, B2, C, and D), which helps link the severity of morphologic changes and clinical signs to appropriate treatment(s).6 Progression of MMVD is hard to predict, but the preclinical stage B is generally longer, often lasting years.3,5,9 When the disease progresses to late stages B2 and C, the survival rates of dogs with CHF drop to 1 to 2 years.9
While canine and human MMVD share many similarities at the molecular and pathophysiological levels,10-12 the factors that contribute to its pathogenesis and progression remain unclear. Both humans and animal studies have linked energy substrate metabolism to cardiac function,13 showing that gene expression and metabolite profiles associated with energy metabolism differ dramatically between healthy and diseased hearts.2 For example, fatty acids are the primary substrate used by mitochondria to generate energy (ATP) for a healthy heart.2 The failing heart must rely on other forms of energy (eg, ketones, glucose, and branched-chain amino acids)2,12 primarily because of decreased mitochondrial oxidative capacity. These energy metabolic changes result in the failing heart becoming less efficient.2,4
System-based approaches and metabolomics analysis are needed to elucidate the mechanism of MMVD development and progression.
Metabolon Insight: Identify Targets and Biomarkers of Mitral Valve Disease Progression
Metabolomic profiling studies on canine MMVD are limited, and direct comparisons of the metabolic profiles between various stages of canine MMVD are lacking. Due to the similarities between canine and human MMVD, insights gleaned from canine studies may have clinical relevance for human MMVD patients.
Data from previous studies led the researchers to construct the current evaluation of canine MMVD, using the Metabolon Global Discovery Panel to elucidate metabolite profile changes. The researchers hypothesized that changes in energy metabolism and remodeling of metabolic machinery would be evident in the dysregulation of energy substrates. Through the holistic approach of metabolomics, the researchers also hoped to draw connections between gut microbial mediators and uremic toxins to the different stages of MMVD. By phenotyping MMVD, identification of potential therapeutic targets and biomarkers of disease progression could be revealed.
The Solution: Metabolomics Compares All Stages of Mitral Valve Disease
The present study4 utilized Metabolon’s untargeted metabolomic analysis to compare all stages of naturally occurring MMVD in dogs. Metabolon’s LC-MS-based metabolomics platform provided high-fidelity analysis to decipher hundreds of discrete metabolites and connect them to key biological pathways in different stages of MMVD, providing a comprehensive view of the underlying biology of the progressive disease.
The Outcome: Mitral Valve Disease Linked to Altered Energy Metabolism
Utilizing a metabolomics approach offered a holistic view of the biological changes underpinning the escalating stages of MMVD.4 Global profiling identified amino acid metabolic reprogramming and renal insufficiency in the pathogenesis of MMVD and congestive heart failure in dogs (Figure 1). Although the strongest trends in the data were detected in CHF dogs, the early-stage B1 dogs still showed shifts in 41% of the significant metabolites. These early changes demonstrate the sensitivity of metabolomics and offer potential biomarkers of disease. Acylcarnitines, tricarboxylic acid (TCA) cycle intermediates, ketone bodies, and creatine accumulated in proportion with MMVD severity, indicating alterations in energy and amino acid metabolism (Figure 2). The concentration of gut-derived metabolites like trimethylamine N-oxide (TMAO) and TMAO-producing precursors were higher in the stage C/D group versus all other groups, as well in stage B2 vs. A. The correlation of gut metabolites with disease state is a point for further investigation, as a microbiome analysis could yield mechanistic answers. In addition to energy remodeling and a potential microbiome connection, kidney function was also dysregulated. Guanidino compounds, indicative of kidney injury, were detected in higher amounts in the MMVD dogs. Further, MMVD dogs had elevated levels of urea, uric acid, and the dipeptide TMAP, an indicator of kidney function. While it is unclear which of these metabolites is integral to MMVD and mitral regurgitation, these changes highlight the cardio-renal link.14
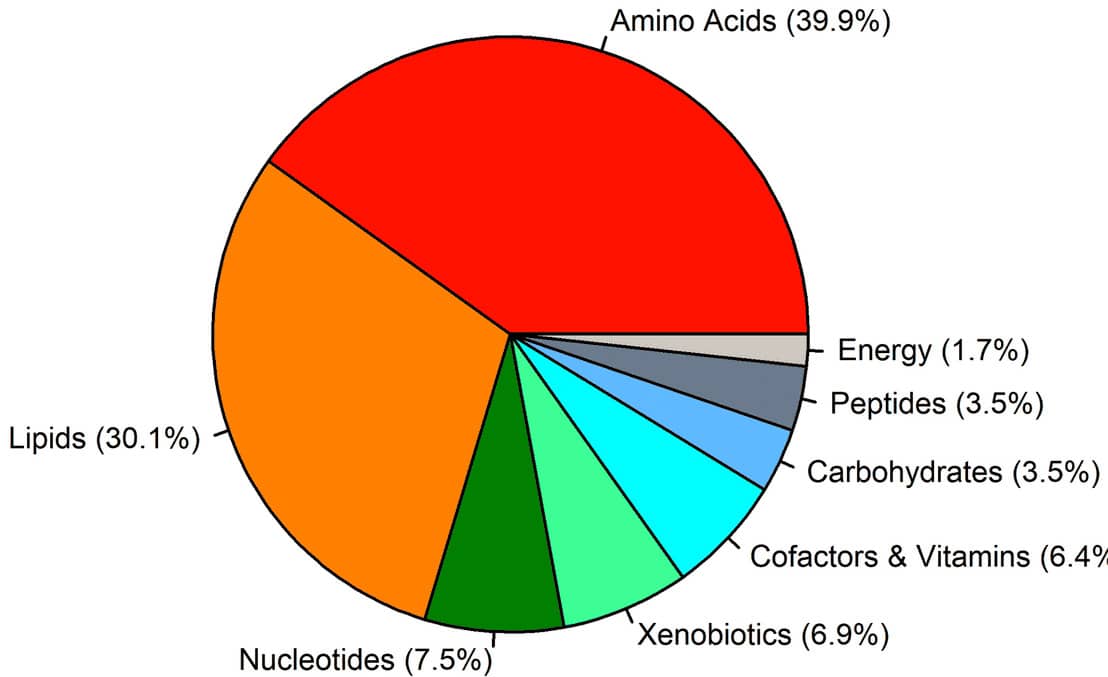
Figure 1. Differential metabolites. The pie chart shows the percentage of classes of the 173 known metabolites that were differentially regulated, 1033 metabolites detected in total.4
Copyright: The copyright holder of Figure 1 is the author/funder of Li, et al., 2021. It is made available under a CC-BY 4.0 International license.
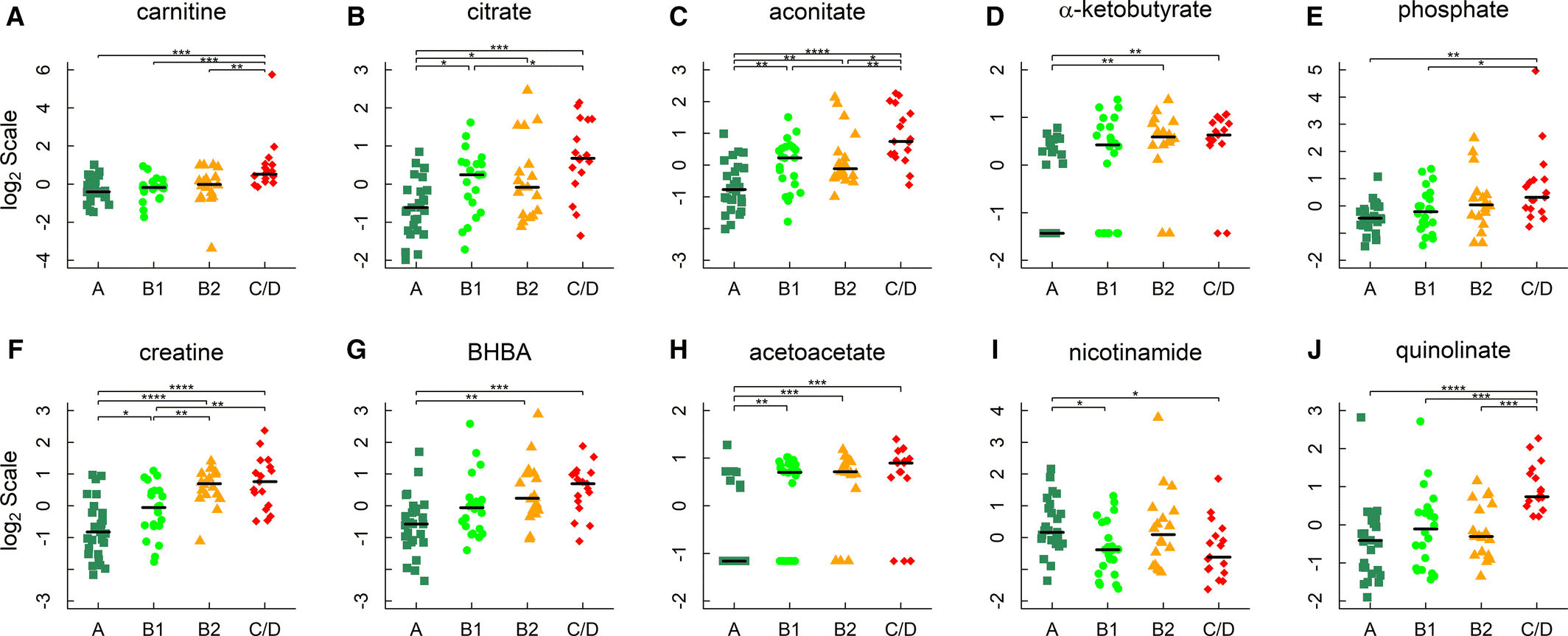
Figure 1. Metabolites in energy metabolism. A through F, Carnitine, citrate, aconitate, α‐ketobutyrate, phosphate, and creatine; (G and H) 3‐hydroxybutyrate/β‐hydroxybutyrate (BHBA) and acetoacetate; and ( I and J) nicotinamide and quinolinate. Horizontal lines denote medians. The metabolites were identified by linear regression analyses and subject to post hoc comparisons with corrections for multiple testing. Adjusted P values: *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.4
Copyright: The copyright holder of Figure 1 is the author/funder of Li, et al., 2021. It is made available under a CC-BY 4.0 International license.
This study demonstrated altered energy metabolism, amino acid metabolic programming, and reduced renal function in canine mitral disease pathology. The changes in many circulating metabolites highlight the potential therapeutic targets and biomarkers that could be used for diagnosis, prognosis, or interventional studies in the future.
References
1. Li, Q. Metabolic Reprogramming, Gut Dysbiosis, and Nutrition Intervention in Canine Heart Disease. Front Vet Sci 9, 791754 (2022).
2. Lopaschuk, G.D., Karwi, Q.G., Tian, R., Wende, A.R. & Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ Res 128, 1487-1513 (2021).
3. Borgarelli, M. & Buchanan, J.W. Historical review, epidemiology and natural history of degenerative mitral valve disease. J Vet Cardiol 14, 93-101 (2012).
4. Li, Q., et al. Metabolomics Analysis Reveals Deranged Energy Metabolism and Amino Acid Metabolic Reprogramming in Dogs With Myxomatous Mitral Valve Disease. J Am Heart Assoc 10, e018923 (2021).
5. Guglielmini, C., et al. Prevalence and risk factors for atrial fibrillation in dogs with myxomatous mitral valve disease. J Vet Intern Med 34, 2223-2231 (2020).
6. Keene, B.W., et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med 33, 1127-1140 (2019).
7. Boswood, A., et al. Longitudinal Analysis of Quality of Life, Clinical, Radiographic, Echocardiographic, and Laboratory Variables in Dogs with Preclinical Myxomatous Mitral Valve Disease Receiving Pimobendan or Placebo: The EPIC Study. J Vet Intern Med 32, 72-85 (2018).
8. O’Brien, M.J., Beijerink, N.J. & Wade, C.M. Genetics of canine myxomatous mitral valve disease. Anim Genet 52, 409-421 (2021).
9. Lord, P., Hansson, K., Kvart, C. & Häggström, J. Rate of change of heart size before congestive heart failure in dogs with mitral regurgitation. J Small Anim Pract 51, 210-218 (2010).
10. Häggström, J., et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med 22, 1124-1135 (2008).
11. Reynolds, C.A., et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: the PREDICT cohort study. J Vet Cardiol 14, 193-202 (2012).
12. Pedersen, H.D. & Häggström, J. Mitral valve prolapse in the dog: a model of mitral valve prolapse in man. Cardiovascular Research 47, 234-243 (2000).
13. Oyama, M.A., et al. Comparative pathology of human and canine myxomatous mitral valve degeneration: 5HT and TGF-β mechanisms. Cardiovascular Pathology 46, 107196 (2020).
14. Pouchelon, J.L., et al. Cardiovascular-renal axis disorders in the domestic dog and cat: a veterinary consensus statement. J Small Anim Pract 56, 537-552 (2015).