Dihydroceramide
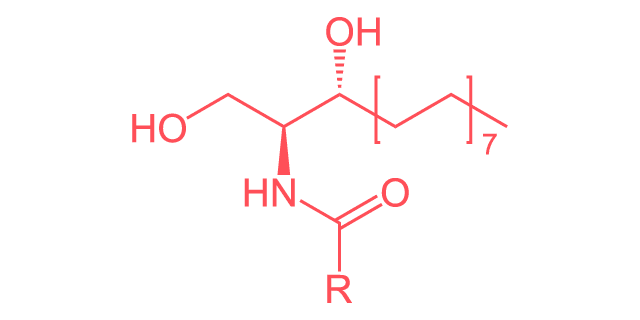
Linear Formula
C19H39NO3
Synonyms
DHC, DCER, DhCer
Share this metabolite
Dihydroceramides are a group of molecules containing a sphingosine backbone and a fatty acyl chain of various potential lengths. They are the precursors of ceramides, central molecules in sphingolipid metabolism. As amphipathic molecules, dihydroceramides have both hydrophobic and hydrophilic properties and are components of the plasma membrane. Together with membrane-bound protein complexes governing their synthesis, dihydroceramides play various roles in cellular stress responses, cell proliferation and cell death, and a variety of diseases.
Dihydroceramide, ceramide synthase, and sphingolipid metabolism
De novo ceramide synthesis begins in the endoplasmic reticulum, where the amino acid L-serine and palmitoyl-CoA are converted to dihydroceramide via a chain of reactions. The final step of the de novo synthesis pathway is the linkage of one of a variety of fatty acids to sphinganine by (dihydro)ceramide synthase (CerS) to produce dihydroceramide1. Six different CerS enzymes can catalyze the reduction of dihydroceramide to ceramide, depending on the length of the fatty acid chain bound to sphinganine. Each enzyme exhibits tissue specificity; for example, CerS2 shows the highest overall expression levels in all tissues, while CerS1 is mostly present in the brain, skeletal muscle, and testis2.
Once dihydroceramide is produced, it can be reduced to ceramide by the enzyme dihydroceramide desaturase (DES), resulting in a pool of ceramides available for de novo sphingolipid biosynthesis in the Golgi apparatus. There are two DES enzymes, each with distinct tissue specificity. Notably, DES1, which is expressed in most tissues, also functions as an oxygen sensor, thus playing a role in managing systemic hypoxia3.
Dihydroceramides and plasma membrane integrity
Dihydroceramides play an important role in cell membrane integrity. Cell membranes containing ceramide are more fluid than membranes containing dihydroceramides as an additional double bond affects their elasticity and packing ability. Additionally, ceramide molecules can stack on top of each other in an anti-parallel way, forming barrel-like structures. These channels can fill with water and perforate the mitochondrial membrane, eventually leaking mitochondrial proteins and leading to the loss of membrane integrity4. In contrast, dihydroceramides do not form such membrane channels and even hinder their formation. Furthermore, altered levels of dihydroceramides change the compositions of membrane lipids of organelles, which provoke responses at cellular and tissue levels.
Dihydroceramides, oxidative stress, and apoptotic cell death
Systemic hypoxia and oxidative stress inhibit DES1, causing dihydroceramide to accumulate in cells. This accumulation is thought to inhibit cell cycle progression as part of the general response to hypoxia5. The accumulation of dihydroceramide not only inhibits cell cycle progression but also inhibits cell growth, affecting cell division rates and potentially leading to cell cycle arrest as part of the cellular response to oxidative stress. Additionally, when cells encounter stress, ceramide levels rise and the ceramide-to-dihydroceramide-ratio shifts6. The resulting perforation of the mitochondrial outer membrane due to the ceramide channels allows for the release of pro-apoptotic proteins, like cytochrome c and adenylate kinase, into the cytosol. Together with protein kinase C and tumor necrosis factor alpha signaling, this abruptly and irreversibly triggers apoptotic cell death; a process implicated in normal physiology but also in various disease processes4.
Dihydroceramides and disease
Several pathological states, including inflammation, tumorigenesis, and the progression of cancer cells and tumor growth, are accompanied by systemic hypoxia and oxidative stress. This has led to a growing body of research into the connections between dihydroceramides and disease, particularly their role in promoting cytotoxic autophagy in cancer cells and inhibiting tumor growth. For example, studies have demonstrated that dihydroceramides, through their regulation of reactive oxygen species and lipid metabolism, impact β-amyloid secretion and production in Alzheimer’s disease3. Additionally, dihydroceramides are involved in modulating immune responses, linking cellular stress to inflammation and the regulation of cytokine-induced inflammation.
Dihydroceramides and cancer cells
The ability of dihydroceramides to promote cell cycle arrest is relevant in cancer research, particularly for patients with breast cancer7. One potential focus is CerS, as their expressions depend on the type, stage, and grade of the breast tumor and the resulting ceramide species having both positive and negative impacts on tumor growth2. Additionally, preliminary studies showed that treating glioma cells with a sphingosine kinase inhibitor as well as human head and neck squamous carcinoma cells with photodynamic therapy led to elevated dihydroceramide levels inhibiting cell growth3. Similarly, inhibiting DES in human neuroblastoma cells prevents the desaturation of dihydroceramide. Accumulated dihydroceramides subsequently promote cytotoxic autophagy, rendering DES another potential target to treat cancer cells8.
Cardiometabolic diseases
In the context of cardiometabolic diseases, the impairment of insulin signaling due to altered dihydroceramide levels has been linked to insulin resistance, further complicating the management of diabetes and cardiovascular complications of obesity. Research studies found the four 18:0, 20:0, 22:0, 24:1 dihydroceramide species to be associated with waist circumference, a marker for lipid accumulation and obesity8. Increased levels of 18:0, 20:0, 22:0, 23:0, and 24:0 dihydroceramides were also shown to be predictors of insulin sensitivity and type 2 diabetes onset3. Studies in rodents further demonstrated that inhibiting DES in adipocytes result in oxidative stress, cell death, and accumulated dihydroceramides and lipids, impairing adipocyte differentiation and function9. Yet, their roles in inducing insulin resistance, insulin sensitivity and hypertension are not well understood.
Infectious diseases
As an integral part of lipid membranes, dihydroceramides play important roles in viral and bacterial infection processes. Pharmacological inhibition of DES1 results in the accumulation of dihydroceramides and their conversion into dihydrosphingomyelin. This molecule rigidifies the cell membrane, and research has shown that this prevents the integration of an HIV-1 docking peptide, blocking HIV-1 infection10.
Several species of oral bacteria, including Porphyromonas gingivalis, Bacteroides spp, Parabacteroides spp, Tannerella spp, and Prevotella spp produce both free and phosphorylated dihydroceramides, such as phosphoethanolamine and phosphoglycerol dihydroceramides. These sphingolipids play important roles in bacterial resistance to reactive oxidative species and in disease progression. For example, phosphorylated dihydroceramides were shown to penetrate osteoclasts and enhance osteoclastogenesis, triggering the breakdown of gum and bone tissue and promoting periodontitis11.
Dihydroceramide in research
As of April 2024, there are over 960 citations for “dihydroceramide” and 860 citations for “dihydroceramide synthesis” in research publications (excluding books and documents) on Pubmed. Several research publications in the last few years have linked this metabolite to a broad range of physiological functions, many of which are discussed here. Hence, any research program seeking to better understand metabolic and neurological health as well as the underlying molecular mechanisms of tumorigenesis and programmed cell death may benefit from the quantitative analysis of this metabolite.
References
- National Library of Medicine, National Center for Biotechnology Information. PubChem Compound Summary, N,N-dimethylarginine (CID 743123831). https://pubchem.ncbi.nlm.nih.gov/compound/123831
- Schnabel R, Blankenberg S, Lubos E, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97(5). doi:10.1161/01.RES.0000181286.44222.61
- Leone A, Moncada S, Vallance P, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339(8793):572-575. doi:10.1016/0140-6736(92)90865-Z
- Achan V, Broadhead M, Malaki M, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscle Thromb Vasc Biol. 2003;23(8):1455-1459. doi:10.1161/01.ATV.0000081742.92006.59
- Zoccali C, Benedetto FA, Maas R, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002;13(2):490-496. doi:10.1681/ASN.V132490
- Wells SM, Buford MC, Migliaccio CT, et al. Elevated Asymmetric Dimethylarginine Alters Lung Function and Induces Collagen Deposition in Mice. Am J Respir Cell Mol Biol. 2009;40(2):179. doi:10.1165/RCMB.2008-0148OC
- Vögeli A, Ottiger M, Meier MA, et al. Asymmetric Dimethylarginine Predicts Long-Term Outcome in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Lung. 2017;195(6):717-727. doi:10.1007/S00408-017-0047-9
- Wu Y, Shen S, Chen J, et al. Metabolite asymmetric dimethylarginine (ADMA) functions as a destabilization enhancer of SOX9 mediated by DDAH1 in osteoarthritis. Sci Adv. 2023;9(6). doi:10.1126/SCIADV.ADE5584
- Singh I, Kim J, Saxena N, et al. Vascular and immunopathological role of Asymmetric Dimethylarginine (ADMA) in Experimental Autoimmune Encephalomyelitis. Immunology. 2021;164(3):602-616. doi:10.1111/IMM.13396
- Mortensen KM, Itenov TS, Hansen MB, et al. Mortality in critical illness: The impact of asymmetric dimethylarginine on survival-A systematic review and meta-analysis. Acta Anaesthesiol Scand. 2019;63(6):708-719. doi:10.1111/AAS.13339
- Aggarwal S, Gross CM, Kumar S, et al. Dimethylarginine dimethylaminohydrolase II overexpression attenuates LPS-mediated lung leak in acute lung injury. Am J Respir Cell Mol Biol. 2014;50(3):614-625. doi:10.1165/RCMB.2013-0193OC
- Sozio E, Hannemann J, Fabris M, et al. The role of asymmetric dimethylarginine (ADMA) in COVID-19: association with respiratory failure and predictive role for outcome. Sci Rep. 2023;13(1):1-10. doi:10.1038/s41598-023-36954-z
